how many electron does nitrogen have|Nitrogen (N) : Tagatay Members of a group typically have similar properties and electron configurations in . Amazon.co.uk Today's Deals Warehouse Deals Outlet Subscribe & Save Vouchers Amazon Prime Prime Video Prime Student Mobile Apps Amazon Pickup Locations 1-48 of over 5,000 results for "outdoor lights" Smart security, inside and out. Shop Ring Cameras . Lezonic Solar Garden Lights Outdoor, 50 LED 7M/23Ft Solar String Lights .
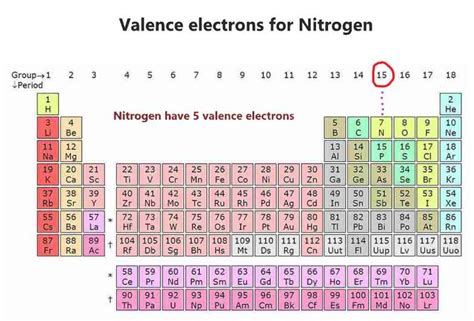
how many electron does nitrogen have,The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), .Members of a group typically have similar properties and electron configurations in .
Electron affinity The energy released when an electron is added to the neutral atom .Nitrogen has seven protons and seven neutrons in its nucleus, and seven electrons in two shells. It is located in group fifteen, period two and block p of the periodic table. .

Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to .how many electron does nitrogen have Nitrogen (N) A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s 2s 2p x2p y2p z. It, therefore, has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired. It has one of the highest electronegativities among the elements (3.04 on the Pauling scale), exceeded only by chlorine (3.16), oxygen (3.44), and fluorine (3.9. Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom .Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only .
Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund’s rule. These three electrons have .Nitrogen (N) [7] — Chemical Element — Periodic Table. ⬇. Physical data. Electronic data. Shells: 2, 5. Orbitals: [He] 2s 2 2p 3. Electronegativity: 3.0, 3.1. 1. Ionization potential: . Nitrogen has 5 electrons in its outermost orbit and it needs 3 more electrons to complete the octet configuration. Thus the nitrogen atom combines with the other nitrogen atom by sharing 3 . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .Nitrogen (N) Nitrogen is the 7th element in the periodic table and has a symbol of N and atomic number of 7. It has an atomic weight of 14.007 and a mass number of 14. Nitrogen has seven protons and seven neutrons in its nucleus, and seven electrons in two shells. It is located in group fifteen, period two and block p of the periodic table.
4th shell can hold 32 electrons. Now the atomic number of Nitrogen (N) is 7. Hence nitrogen element has the electrons arrangement 2, 5. This electron arrangement indicates that the . The standard atomic mass of nitrogen is 14.006 and its symbol is ‘N’. The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons in the last shell after the electron configuration of nitrogen is called the valence electrons of nitrogen. The last shell of nitrogen has five electrons.

Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration .
Protons. A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one ( + 1) and a mass of 1 atomic mass unit (amu), which is about 1.67 × 10 − 27 kilograms.From the perspective of living systems two of the most interesting elements are nitrogen and oxygen. Carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. Nitrogen has seven electrons: two core and five valence: 1s2, 2s2, 2px1, 2py1, 2pz1. So if you are following the rules, you might well assume . 7 electrons, 7 protons, and 7 neutrons. Nitrogen-14 is actually an isotope of nitrogen, so right from the start, you can say that it is a neutral atom. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. You know that nitrogen-14 has 7 protons in the nucleus because it is an isotope of nitrogen, . Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund’s rule. These three electrons have unpaired spins. Oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each . The nitrogen atom has a total of 7 electrons so, we have to put 7 electrons in orbitals. The electrons will be placed in different orbitals according to the energy level: [1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f]. Now, Nitrogen electron configuration N (7) = 1s 2 2s 2 2p 3 (complete configuration).Answer to: An atom of nitrogen has seven protons in its nucleus. How many electrons does it have? By signing up, you'll get thousands of.
How many valence electrons does Nitrogen have? Nitrogen has 5 electrons in its outer shell and 2 electrons in its inner shell. Hence it has a total of 7 electrons. The valency of nitrogen .Nitrogen (N) 9 years ago. The valence electrons of nitrogen in its compounds are all sp³ hybridized orbitals. The formal charge on N is usually -1 for an anion, 0 for a neutral compound, and +1 in cations. A nitrogen atom with a formal charge of -3 would correspond to a .
Nitrogen - Atomic Number. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. . (group 2, or alkaline earth metals) of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure. 24.305 .
The correct electronic structure for nitrogen is 1s 2 2s 2 2p 3, with two electrons in the 1s orbital, two electrons in the 2s orbital, and three electrons in the 2p orbital. The atomic number of nitrogen is 7, which means its atom has seven electrons. Therefore, the electron configuration of nitrogen is 1s2 2s2 2p3.
Ammonia. This is an ammonia molecule with the formula, NH3. Scientists use the name "ammonia", the same way they call H2O "water". In this compound, three hydrogen (H) atoms share their electrons with the nitrogen (N) atom. This way the nitrogen has a filled outer shell and the hydrogens have two electrons to fill their shells. Nitrogen's atomic number is 7. That means that it has 7 positively charged protons in the nucleus. To be neutral, nitrogen must then also have 7 negatively charged electrons in its electron cloud.Answer to: How many electrons does nitrogen have? By signing up, you'll get thousands of step-by-step solutions to your homework questions. You can.
how many electron does nitrogen have|Nitrogen (N)
PH0 · Nitrogen (N) [7] — Chemical Element — Periodic Table
PH1 · Nitrogen (N)
PH2 · Nitrogen
PH3 · Electron Configuration for Nitrogen (N)
PH4 · Chemistry of Nitrogen (Z=7)
PH5 · 8.9.2: Chemistry of Nitrogen (Z=7)
PH6 · 6.4 Electronic Structure of Atoms (Electron Configurations)